United Kingdom (EN)
Select your region or country.
Navigating the Evolution of Light-Sheet Microscopy Through Time and Technology
Journey through the history of scientific exploration, where light-sheet microscopy meets the complexities of life sciences. Over the past three decades, microscopic techniques have advanced remarkably, fueled by the innovation of scientific CMOS cameras.
About light-sheet microscopy
Light microscopy is a core technology in life science research. Since its early days, it has evolved with a wide range of microscopic techniques, driven by the rapid development of optoelectronic detectors and the widespread availability of laser-based light sources. This development is clear when observing the distinctive design of light-sheet microscopes, which varies greatly from the standard microscope design that has remained for centuries.
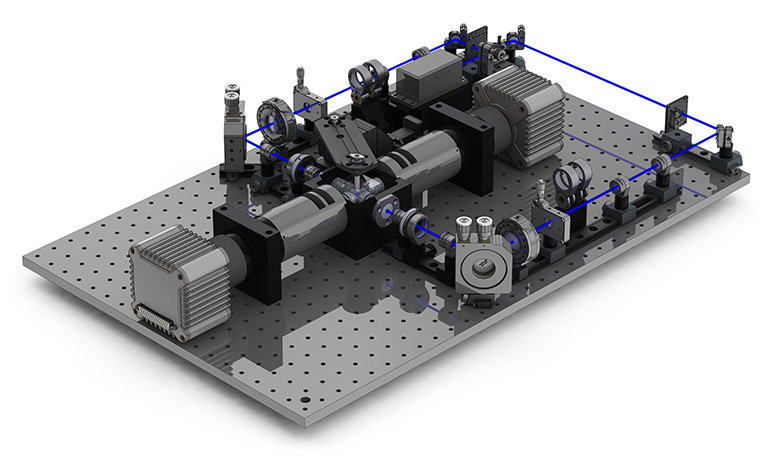
© Fig. 1: Light-sheet microscope, OpenSPIM concept configurations.
The fundamental concept underlying all light-sheet microscopes is that the sample is illuminated with a thin sheet of light (hence the name), typically perpendicular to the observation axis. This decoupling of the illumination and the detection offers greater freedom in design compared to typical epi- or transillumination designs.
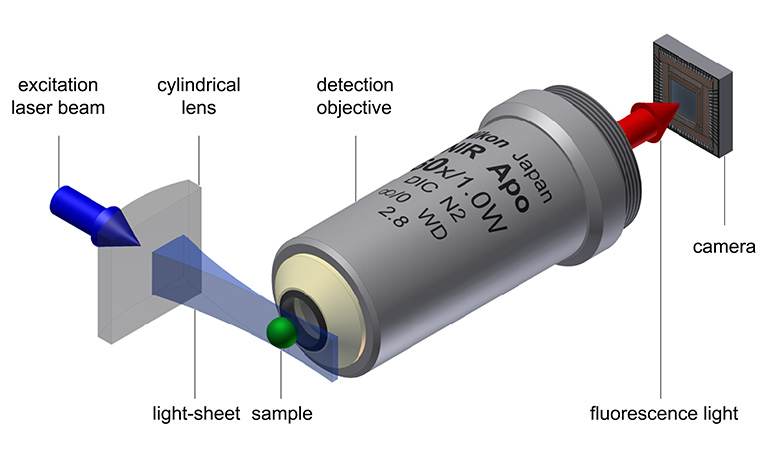
© Fig. 2: Light-sheet microscopy principle, based on Jan Krieger, CC BY-SA 3.0.
When combined with fluorescence staining, light-sheet microscopy offers several advantages, including 3D resolution and enhanced contrast as there is no out of-focus light generated. This gentle technique preserves both the signal thanks to low photobleaching and the sample integrity due to low phototoxicity. With camera-based detection, acquisition can be swift, limited only by the maximum frame rate of the camera. The flexible design of light-sheet microscopes allows specialization for specific samples, ranging from a few centimeters to the single-cell level (Girkin, 2018 [1]).
While those advantages encourage the replacement of standard microscopes, it must be noted that they come with some disadvantages, such as sample mounting, which may hinder certain applications with established protocols. The flexibility in instrument design, which certainly is an advantage on many occasions, might add unwanted complexity to some experiments. Additionally, while the resolution can be diffraction-limited, the highest resolution is still achieved with standard microscopes or with super-resolution approaches, despite demonstrations of combining light-sheet microscopy with super-resolution techniques.
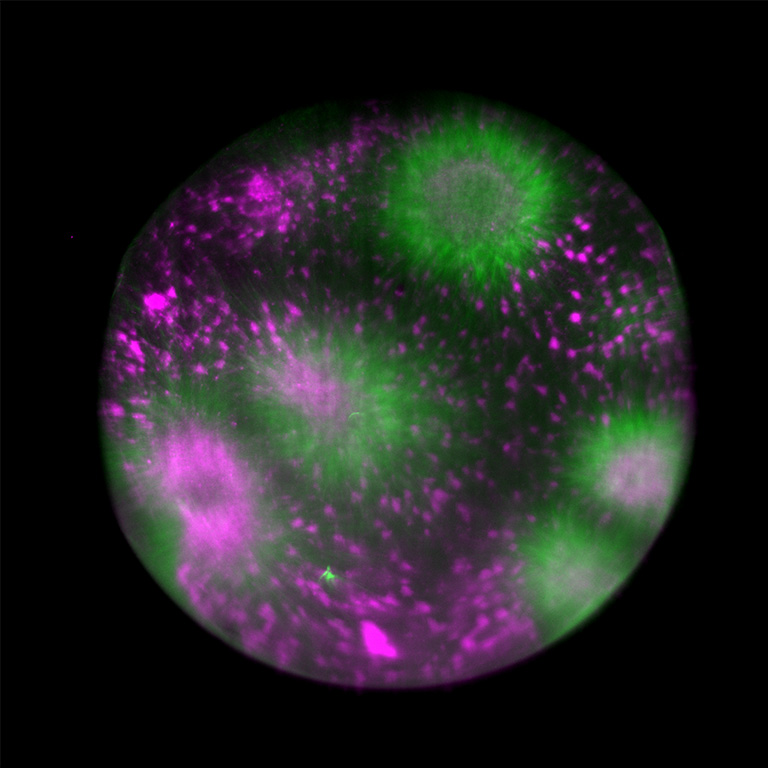
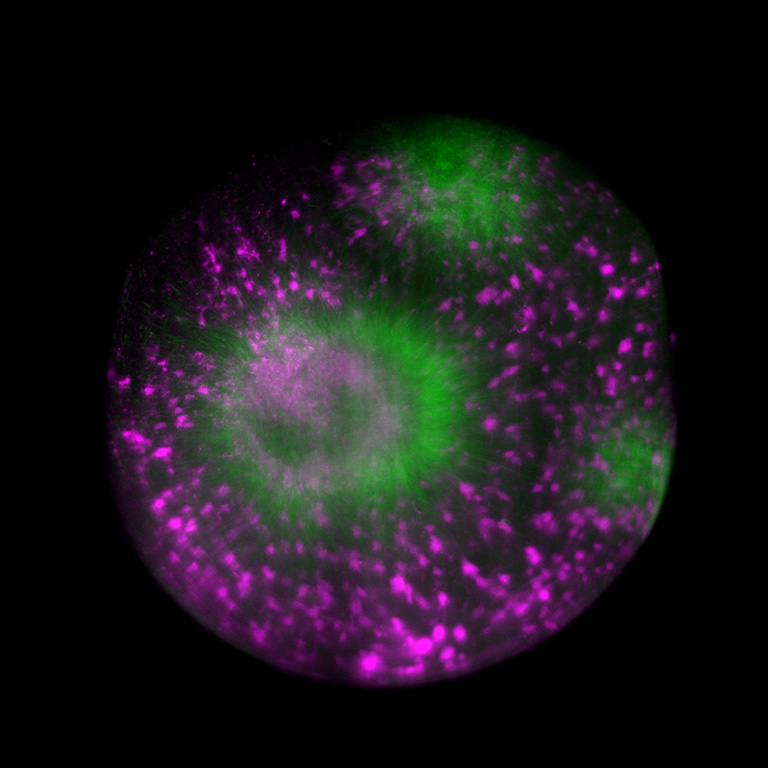
© Fig. 3: Tubulin assembly in cytoplasmic droplet, acquired on Viventis LS1 light-sheet microscope with ORCA-Fusion BT Digital CMOS camera.
Source: Dr. Michael Riedl (Jan Bruges Group) and Dr. Vanessa Carlos (PoL Microscopy), Cluster of Excellence “Physics of Life”, TU Dresden.
The origins of light-sheet microscopy
While the basic idea of illumination with a light-sheet is more than a century old, the technique only gained prominence 20 years ago. The success is attributed not only to the widespread availability of laser illumination but also to the advent of fast digital cameras enabling 3D reconstruction of images.
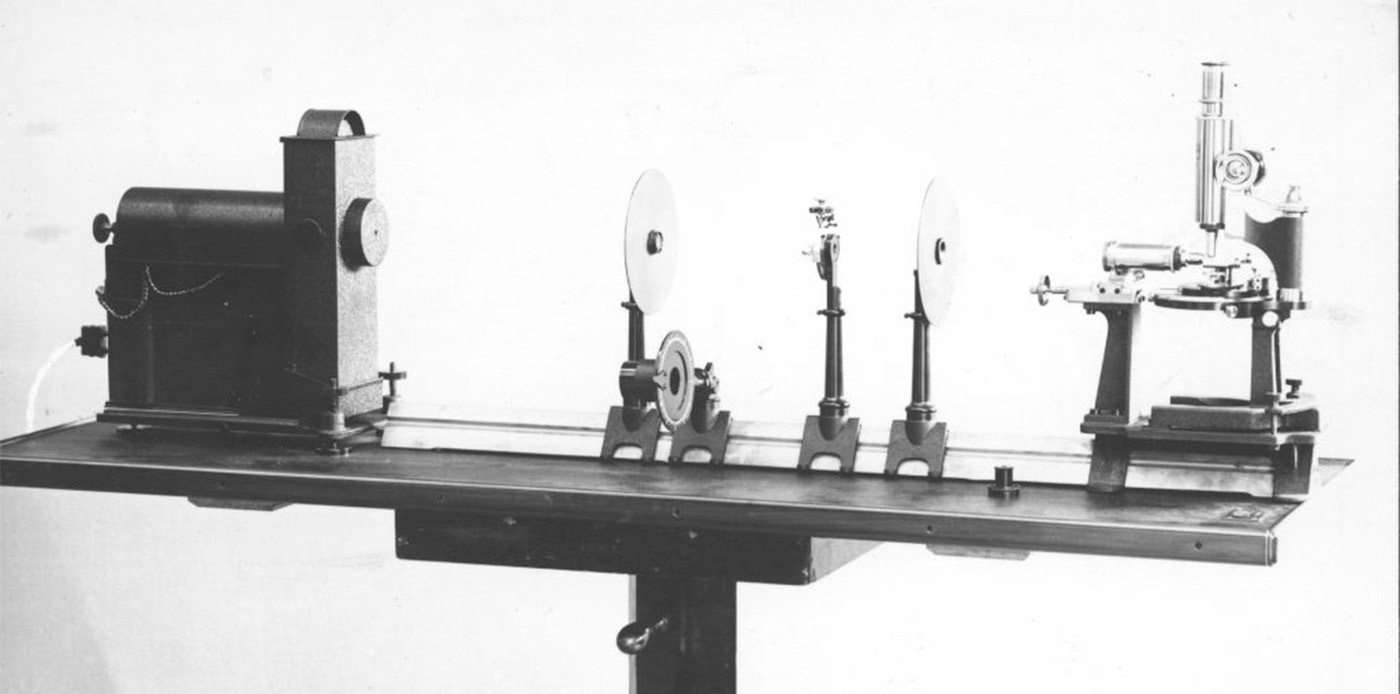
Delving into history, we trace the origins back to 1903 when Siedentopf & Zsigmondy pioneered the first light-sheet microscope (Siedentopf. H. and Zsigmondy, 1903 [2]). Their objective, analyzing sub-resolution gold particles, led to a groundbreaking Nobel prize in 1925, the first awarded for a microscopy technique. Their so called “Ultramicroscope” (see below) was a dark field light-sheet setup, designed to visualize particles as small as 4 nm. Apart from being the first demonstrated light-sheet microscope, they also started colloid research, which some view as the foundation of the field of nanotechnology (Mappes, 2012 [3]).
Invention of the ultramicroscope
© Henry Siedentopf and Richard A. Zsigmondy; ZEISS Microscopy
In 1903 Ernst Abbe retires from the management, handicapped by severe health problems. He lives to see in this year the limits of classical microscopy finally exceeded by the ultramicroscope, an invention by Henry Siedentopf and Richard Zsigmondy, which makes submicroscopical colloids visible. This is actually the first published account of a very simple version of a light-sheet fluorescence microscope (or LSFM) in which light of an arc lamp was projected through a slit aperture to observe gold particles.
Nearly a century later, Voie et al. revived the technique and applied it for the first time to a biological sample, the inner ear cochlea of a guinea pig, using tissue-clearing methods to reveal the inner structure of the tissues (Voie, 1993 [4]). Interestingly, they chose an application (imaging of cleared tissue) which blossomed into a major field 20 years later with the advent of modern tissue-clearing protocols.
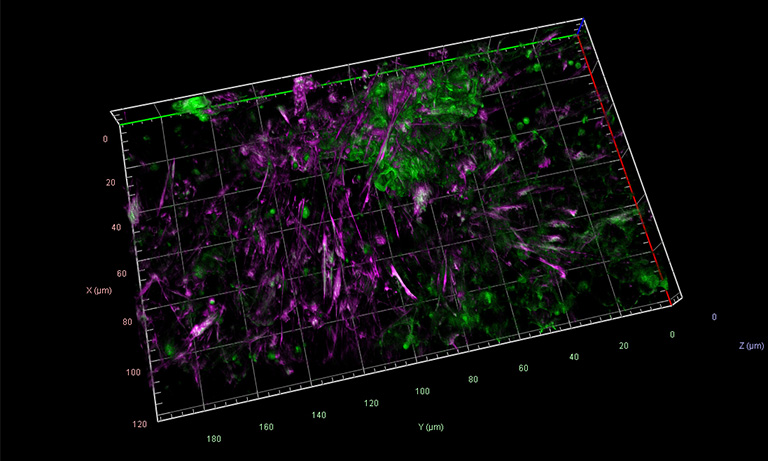
© Fig. 4: Epithelial cells stained for E-Cadherin and Actin, imaged on Zeiss Lattice Light-Sheet 7 with ORCA® Fusion cameras. Source: Dr. Anastasiia Gabrielyan (Natalie Dye Group) and Dr. Bert Nitzsche (PoL Microscopy), Cluster of Excellence “Physics of Life”, TU Dresden.
Modern-day light-sheet microscopy
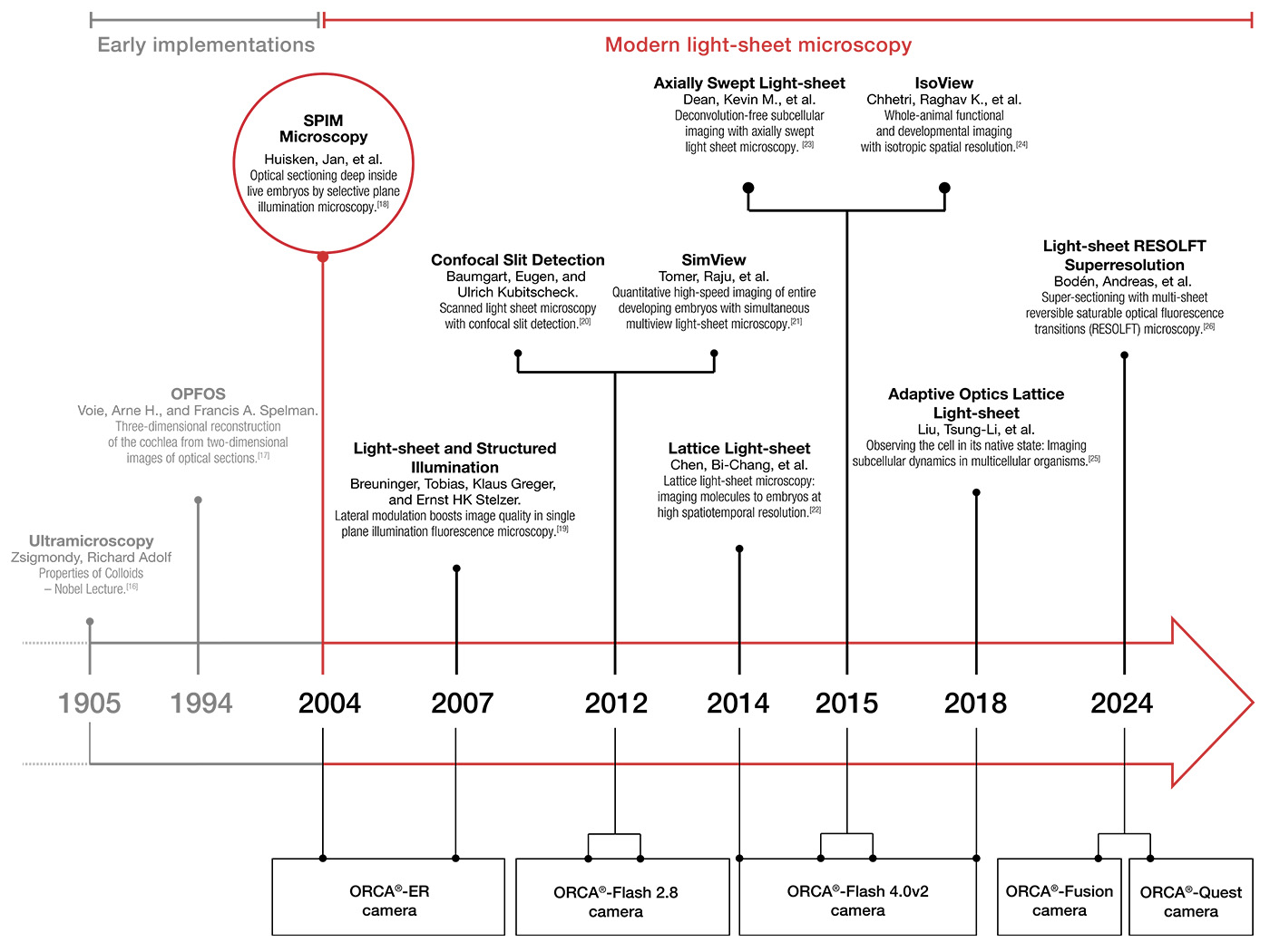
The year that light-sheet microscopy finally took off was 2004: Jan Huisken developed selective plane illumination microscopy (SPIM), enhancing earlier light-sheet microscopes by incorporating a microscope objective to the illumination arm and a Hamamatsu Photonics ORCA-ER B/W CCD digital camera. This innovative approach improved the 3D resolution and reduced photobleaching, showcasing low phototoxicity and high speed in observing Drosophila melanogaster (fruit fly) embryogenesis and medaka (Japanese rice fish) embryos’ heartbeat (Huisken, 2004 [5]).
During the next decade, light-sheet microscopy became a recognized tool in developmental biology, witnessing advancements like digital scanned light-sheet and creative implementations like “oblique plane microscopy”. It combined the advantages of light-sheet microscopy with classical microscope bodies and hence brought the light-sheet advantages to standard samples on glass slides (Dunsby, 2008 [6]).
Technological acceleration in the early 2010s saw the introduction of scientific CMOS cameras (sCMOS), surpassing previous EMCCDs with more pixels at higher speeds and increased sensitivity – an ideal combination for large, sensitive specimens. Hamamatsu’s sCMOS cameras, particularly Flash 2.8 and 4.0, played a significant role in this shift. Even the initially perceived drawback of CMOS cameras, the rolling shutter, had been converted into an advantage in light-sheet microscopy. The synchronization of the excitation beam in digitally scanned light-sheets with the rolling shutter contributed to reduced stray light, increased contrast, and axial resolution (Baumgart, 2012 [7]). The rolling shutter could also be synchronized with a tunable lens in ‘axially swept light-sheet microscopy’ for a uniform light-sheet across large field of views (Dean K. M., 2015 [8]).
To simplify the synchronization of the rolling shutter with external equipment, Hamamatsu developed the patented ‘Light-sheet readout mode’. With the release of the Flash 4.0 V2 in 2013, sCMOS technology reached its maturity. Since this technology appeared – and potentially sparked the development burst of light-sheet microscopy, the Flash 4.0 found widespread use in high-end light-sheets, including those by Nobel laureate Eric Betzig (Chen B. C., 2014 [9])) (Liu, 2018 [10]), Philip Keller’s sophisticated setups (MuVi SPIM, IsoView) (Krzic, 2012 [11]) (Chhetri, 2015 [12]), Reto Fiolka’s innovative microscopy techniques - axially swept light-sheet microscope and many more (Dean K. M., 2015 [13]), Illaria Testa’s super resolution and light-sheet combinations (Bodén, 2024 [14]) and various others.
Subsequent developments brought forth a whole range of sCMOS cameras, with numerous research projects utilizing Hamamatsu’s ORCA® series. In fact, over the last four years, more than 1000 scientific papers* have been published using an ORCA® sCMOS camera. Each camera offers specific features such as different dynamic ranges, shutter functionalities, and sensor dimensions, catering to the unique demands of diverse research endeavors.
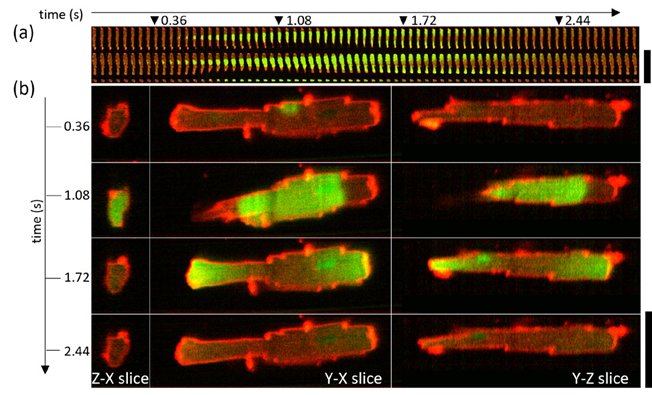
© Fig. 5: Spontaneous calcium wave in a live cardiomyocyte cell imaged at 25 volumes per second. (a) shows central volume planes in xy, xz and yz orientation, while (b) shows the expanded views of the datapoints indicated with triangles in (a). Source: Dr. Hugh Sparks and Dr. Christopher Dunsby, Imperial College London, as published.[15]
The synergy of scientific research and technological advancements
As we navigate the rich history of light-sheet microscopy, a story of constant innovation unfolds. The technique, revolutionized by pioneers like Siedentopf & Zsigmondy in 1903 and revitalized by Voie et al. in 1993, reached a transformative moment in 2004 with the advent of selective plane illumination microscopy (SPIM). At the heart of SPIM's success was Hamamatsu Photonics' ORCA®-ER B/W CCD digital camera, bringing forth 3D resolution, low photobleaching, and high speed to the forefront. The early 2010s marked a technological peak with the maturity of sCMOS cameras, which was encapsulated by Hamamatsu's Flash 4.0 V2, propelling light-sheet microscopy to new heights. This evolution paved the way for diverse research projects utilizing Hamamatsu's ORCA® series.
Additionally, today, there is a wide range of options for light-sheet setups, ranging from very basic Do-It-Yourself projects like OpenSPIM with step-by-step tutorials to more advanced open hardware projects such as the mesoSPIM project, along with commercial solutions. Remarkably, the flexibility of some of these approaches allows for building the microscope around the sample, rather than fitting the sample to the microscope.
As we anticipate the future of light-sheet microscopy, we predict the continuous collaboration between technology and scientific discovery, with each milestone marking a step closer to unraveling the mysteries of life sciences.
20 years of light-sheet microscopy innovation with Hamamatsu
References
[1] Girkin, J. M. (2018). The light-sheet microscopy revolution. Journal of Optics.
[2] Siedentopf. H. and Zsigmondy, R. (1903). Über Sichtbarmachung und Größenbestimmung ultramikroskopischer Teilchen, mit besonderer Anwendung auf Rubingläser. Analen der Physik, 692 - 702.
[3] Mappes, T. J. (2012). The invention of immersion ultramicroscopy in 1912—the birth of nanotechnology? Angewandte Chemie International Edition, 11208-11212.
[4] Voie, A. H. (1993). Orthogonal-plane fluorescence optical sectioning: Three-dimensional imaging of macroscopic biological specimens. Journal of microscopy, 229 - 236.
[5] Huisken, J. e. (2004). Optical sectioning deep inside live embryos by selective plane illumination microscopy. Science, 1007-1009.
[6] Dunsby, C. (2008). Optically sectioned imaging by oblique plane microscopy. Optics express, 20306 - 20316.
[7] Baumgart, E. &. (2012). Scanned light-sheet microscopy with confocal slit detection. Optics express, 21805 - 21814.
[9] Chen, B. C. (2014). Lattice light-sheet microscopy: imaging molecules to embryos at high spatiotemporal resolution. Science.1257998.
[10] Liu, T.-L. e. (2018). Observing the cell in its native state: Imaging subcellular dynamics in multicellular organisms. Science, 6386.
[11] Krzic, U. e. (2012). Multiview light-sheet microscope for rapid in toto imaging. Nature methods, 730-733.
[12] Chhetri, R. K. (2015). Whole-animal functional and developmental imaging with isotropic spatial resolution. Nature Methods, 1171-1178.
[13] Dean, K. M. (2015). Deconvolution-free subcellular imaging with axially swept light-sheet microscopy. Biophysical journal, 2807 - 2815.
[14] Bodén, A. e. (2024). Super-sectioning with multi-sheet reversible saturable optical fluorescence transitions (RESOLFT) microscopy. Nature Methods, 1-7.
[15] Sparks, Hugh, et al. "Development a flexible light-sheet fluorescence microscope for high-speed 3D imaging of calcium dynamics and 3D imaging of cellular microstructure." Journal of Biophotonics 13.6 (2020): e201960239.
[16] Zsigmondy, Richard Adolf (December 11, 1926). "Properties of Colloids – Nobel Lecture". Nobel Lectures, Chemistry 1922-1941.
[17] Voie, Arne H., and Francis A. Spelman. "Three-dimensional reconstruction of the cochlea from two-dimensional images of optical sections." Comput- erized medical imaging and graphics 19.5 (1995): 377-384.
[18] Huisken, Jan, et al. "Optical sectioning deep inside live embryos by selective plane illumination microscopy." Science 305.5686 (2004): 1007-1009.
[19] Breuninger, Tobias, Klaus Greger, and Ernst HK Stelzer. "Lateral modulation boosts image quality in single plane illumination fluorescence microscopy." Optics letters 32.13 (2007): 1938-1940.
[20] Baumgart, Eugen, and Ulrich Kubitscheck. "Scanned light-sheet microscopy with confocal slit detection." Optics express 20.19 (2012): 21805-21814.
[21] Tomer, Raju, et al. "Quantitative high-speed imaging of entire developing embryos with simultaneous multiview light-sheet microscopy." Nature meth- ods 9.7 (2012): 755-763.
[22] Chen, Bi-Chang, et al. "Lattice light-sheet microscopy: imaging molecules to embryos at high spatiotemporal resolution." Science 346.6208 (2014): 1257998.
[23] Dean, Kevin M., et al. "Deconvolution-free subcellular imaging with axially swept light-sheet microscopy." Biophysical journal 108.12 (2015): 2807-2815.
[24] Chhetri, Raghav K., et al. "Whole-animal functional and developmental imaging with isotropic spatial resolution." Nature methods 12.12 (2015): 1171-1178.
[25] Liu, Tsung-Li, et al. "Observing the cell in its native state: Imaging subcellular dynamics in multicellular organisms." Science 360.6386 (2018): eaaq1392.
[26] Bodén, Andreas, et al. "Super-sectioning with multi-sheet reversible saturable optical fluorescence transitions (RESOLFT) microscopy." Nature Methods (2024): 1-7.
*The research was made on Meltwater Media Monitoring from the year 2000 to 2023.
- Confirmation
-
It looks like you're in the . If this is not your location, please select the correct region or country below.
You're headed to Hamamatsu Photonics website for GB (English). If you want to view an other country's site, the optimized information will be provided by selecting options below.
In order to use this website comfortably, we use cookies. For cookie details please see our cookie policy.
- Cookie Policy
-
This website or its third-party tools use cookies, which are necessary to its functioning and required to achieve the purposes illustrated in this cookie policy. By closing the cookie warning banner, scrolling the page, clicking a link or continuing to browse otherwise, you agree to the use of cookies.
Hamamatsu uses cookies in order to enhance your experience on our website and ensure that our website functions.
You can visit this page at any time to learn more about cookies, get the most up to date information on how we use cookies and manage your cookie settings. We will not use cookies for any purpose other than the ones stated, but please note that we reserve the right to update our cookies.
1. What are cookies?
For modern websites to work according to visitor’s expectations, they need to collect certain basic information about visitors. To do this, a site will create small text files which are placed on visitor’s devices (computer or mobile) - these files are known as cookies when you access a website. Cookies are used in order to make websites function and work efficiently. Cookies are uniquely assigned to each visitor and can only be read by a web server in the domain that issued the cookie to the visitor. Cookies cannot be used to run programs or deliver viruses to a visitor’s device.
Cookies do various jobs which make the visitor’s experience of the internet much smoother and more interactive. For instance, cookies are used to remember the visitor’s preferences on sites they visit often, to remember language preference and to help navigate between pages more efficiently. Much, though not all, of the data collected is anonymous, though some of it is designed to detect browsing patterns and approximate geographical location to improve the visitor experience.
Certain type of cookies may require the data subject’s consent before storing them on the computer.
2. What are the different types of cookies?
This website uses two types of cookies:
- First party cookies. For our website, the first party cookies are controlled and maintained by Hamamatsu. No other parties have access to these cookies.
- Third party cookies. These cookies are implemented by organizations outside Hamamatsu. We do not have access to the data in these cookies, but we use these cookies to improve the overall website experience.
3. How do we use cookies?
This website uses cookies for following purposes:
- Certain cookies are necessary for our website to function. These are strictly necessary cookies and are required to enable website access, support navigation or provide relevant content. These cookies direct you to the correct region or country, and support security and ecommerce. Strictly necessary cookies also enforce your privacy preferences. Without these strictly necessary cookies, much of our website will not function.
- Analytics cookies are used to track website usage. This data enables us to improve our website usability, performance and website administration. In our analytics cookies, we do not store any personal identifying information.
- Functionality cookies. These are used to recognize you when you return to our website. This enables us to personalize our content for you, greet you by name and remember your preferences (for example, your choice of language or region).
- These cookies record your visit to our website, the pages you have visited and the links you have followed. We will use this information to make our website and the advertising displayed on it more relevant to your interests. We may also share this information with third parties for this purpose.
Cookies help us help you. Through the use of cookies, we learn what is important to our visitors and we develop and enhance website content and functionality to support your experience. Much of our website can be accessed if cookies are disabled, however certain website functions may not work. And, we believe your current and future visits will be enhanced if cookies are enabled.
4. Which cookies do we use?
There are two ways to manage cookie preferences.
- You can set your cookie preferences on your device or in your browser.
- You can set your cookie preferences at the website level.
If you don’t want to receive cookies, you can modify your browser so that it notifies you when cookies are sent to it or you can refuse cookies altogether. You can also delete cookies that have already been set.
If you wish to restrict or block web browser cookies which are set on your device then you can do this through your browser settings; the Help function within your browser should tell you how. Alternatively, you may wish to visit www.aboutcookies.org, which contains comprehensive information on how to do this on a wide variety of desktop browsers.
5. What are Internet tags and how do we use them with cookies?
Occasionally, we may use internet tags (also known as action tags, single-pixel GIFs, clear GIFs, invisible GIFs and 1-by-1 GIFs) at this site and may deploy these tags/cookies through a third-party advertising partner or a web analytical service partner which may be located and store the respective information (including your IP-address) in a foreign country. These tags/cookies are placed on both online advertisements that bring users to this site and on different pages of this site. We use this technology to measure the visitors' responses to our sites and the effectiveness of our advertising campaigns (including how many times a page is opened and which information is consulted) as well as to evaluate your use of this website. The third-party partner or the web analytical service partner may be able to collect data about visitors to our and other sites because of these internet tags/cookies, may compose reports regarding the website’s activity for us and may provide further services which are related to the use of the website and the internet. They may provide such information to other parties if there is a legal requirement that they do so, or if they hire the other parties to process information on their behalf.
If you would like more information about web tags and cookies associated with on-line advertising or to opt-out of third-party collection of this information, please visit the Network Advertising Initiative website http://www.networkadvertising.org.
6. Analytics and Advertisement Cookies
We use third-party cookies (such as Google Analytics) to track visitors on our website, to get reports about how visitors use the website and to inform, optimize and serve ads based on someone's past visits to our website.
You may opt-out of Google Analytics cookies by the websites provided by Google:
https://tools.google.com/dlpage/gaoptout?hl=en
As provided in this Privacy Policy (Article 5), you can learn more about opt-out cookies by the website provided by Network Advertising Initiative:
http://www.networkadvertising.org
We inform you that in such case you will not be able to wholly use all functions of our website.
Close